In Vehicle Ammonia Cracking
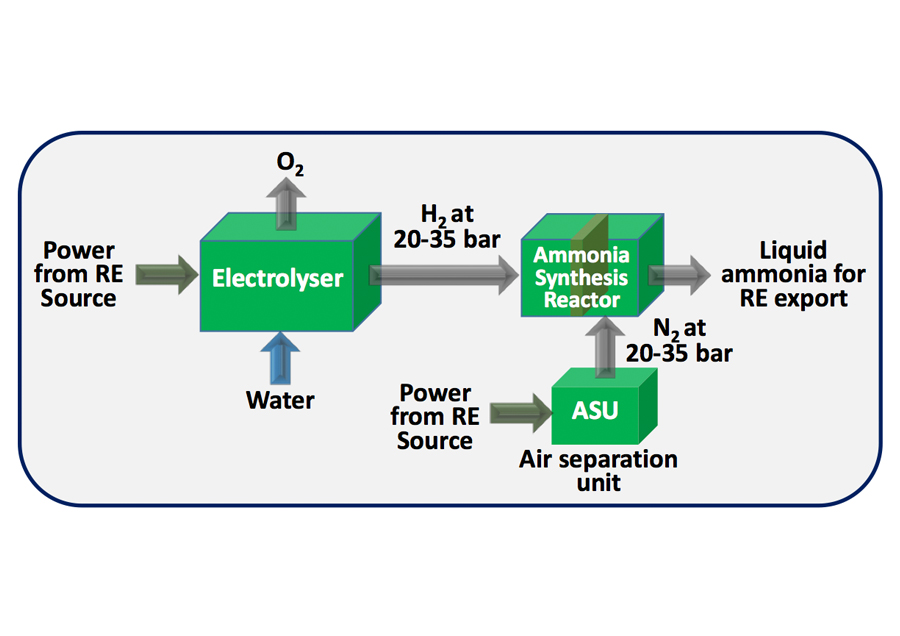
In vehicle ammonia cracking is a critical process for the on-board production of hydrogen for fuel cell vehicles. Ammonia (NH3) is a promising hydrogen carrier due to its high energy density, ease of storage and transportation, and relatively low production costs. The cracking of ammonia into hydrogen and nitrogen is a complex process that requires careful consideration of catalysts, reaction conditions, and system design.
Introduction to Ammonia Cracking

Ammonia cracking is a chemical reaction that involves the decomposition of ammonia into hydrogen and nitrogen. The reaction is highly endothermic, meaning it requires a significant amount of energy to proceed. The overall reaction is: 2NH3 → 3H2 + N2. This reaction is typically carried out over a catalyst, such as a metal or metal oxide, at elevated temperatures (between 500°C to 900°C) and pressures (between 1 atm to 10 atm).
Catalysts for Ammonia Cracking
The choice of catalyst is critical for efficient ammonia cracking. Ruthenium (Ru) and rhodium (Rh) are commonly used catalysts due to their high activity and selectivity for hydrogen production. However, these noble metals are expensive and can be prone to deactivation. Transition metal oxides, such as iron oxide (Fe2O3) and cobalt oxide (Co3O4), have also been investigated as potential catalysts due to their lower costs and improved stability.
| Catalyst | Activity (mol H2/g cat/h) | Selectivity (% H2) |
|---|---|---|
| Ru/Al2O3 | 120 | 95 |
| Rh/Al2O3 | 100 | 92 |
| Fe2O3 | 50 | 85 |
| Co3O4 | 40 | 80 |

Reaction Conditions and System Design

The reaction conditions, such as temperature, pressure, and ammonia flow rate, significantly impact the efficiency and productivity of the ammonia cracking process. Temperature is a critical parameter, as it influences the reaction kinetics, catalyst activity, and hydrogen yield. Pressure also plays a role, as it affects the reaction equilibrium and hydrogen purity. The system design, including the reactor configuration, heat management, and gas separation, must be carefully optimized to achieve high efficiency and productivity.
Reactor Configurations
Various reactor configurations have been investigated for in vehicle ammonia cracking, including fixed bed reactors, fluidized bed reactors, and microreactors. Each configuration has its advantages and disadvantages, and the choice of reactor design depends on the specific application and requirements.
- Fixed bed reactors: simple design, low cost, but prone to catalyst deactivation and limited heat transfer.
- Fluidized bed reactors: improved heat transfer, catalyst stability, but complex design and high energy consumption.
- Microreactors: compact design, high efficiency, but limited scalability and high manufacturing costs.
What are the advantages of using ammonia as a hydrogen carrier?
+Ammonia has several advantages as a hydrogen carrier, including high energy density, ease of storage and transportation, and relatively low production costs. Additionally, ammonia can be produced from renewable energy sources, making it a promising option for a sustainable energy future.
What are the challenges associated with in vehicle ammonia cracking?
+The challenges associated with in vehicle ammonia cracking include the development of efficient and cost-effective catalysts, optimization of reaction conditions and system design, and ensuring safe and reliable operation. Additionally, the storage and handling of ammonia require special considerations due to its toxic and corrosive properties.
In vehicle ammonia cracking is a complex process that requires careful consideration of catalysts, reaction conditions, and system design. While there are challenges associated with this technology, the potential benefits of using ammonia as a hydrogen carrier make it an attractive option for fuel cell vehicles. Further research and development are needed to overcome the technical and economic barriers and to commercialize this technology.
