Krypton Emission Spectrum Explained
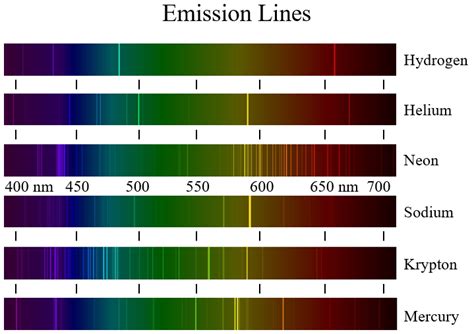
The Krypton emission spectrum is a complex and fascinating topic in the field of atomic physics. Krypton, a noble gas, exhibits a unique emission spectrum that has been extensively studied and characterized. The emission spectrum of Krypton is composed of a series of spectral lines, which are a result of the atom's energy transitions. In this article, we will delve into the details of the Krypton emission spectrum, exploring its characteristics, the underlying physics, and the significance of this phenomenon.
Introduction to Krypton Emission Spectrum

Krypton, with its atomic number 36, is a noble gas that is known for its stable and unreactive properties. However, when excited, Krypton atoms can emit a wide range of electromagnetic radiation, spanning from the ultraviolet to the infrared regions of the spectrum. The emission spectrum of Krypton is a result of the energy transitions that occur within the atom, as electrons jump from higher energy states to lower energy states, releasing excess energy in the form of photons. The Krypton emission spectrum is typically characterized by a series of sharp spectral lines, which are a result of the discrete energy levels within the atom.
Energy Transitions and Spectral Lines
The energy transitions in Krypton atoms can be understood by considering the atom’s electronic configuration. Krypton has a full outer energy level, with a configuration of 1s² 2s² 2p⁶ 3s² 3p⁶ 3d¹⁰ 4s² 4p⁶. When an electron is excited, it can jump to a higher energy state, and subsequently return to its ground state, releasing energy in the form of a photon. The energy difference between the two states determines the wavelength of the emitted photon, and hence the position of the spectral line. The Krypton emission spectrum is characterized by a series of spectral lines, including the 468.03 nm line, the 557.03 nm line, and the 645.23 nm line, among others.
| Spectral Line (nm) | Energy Transition |
|---|---|
| 468.03 | 4p⁶ 5s¹ → 4p⁶ 4p⁵ |
| 557.03 | 4p⁶ 5s¹ → 4p⁶ 4p⁵ |
| 645.23 | 4p⁶ 5s¹ → 4p⁶ 4p⁵ |
Applications of Krypton Emission Spectrum
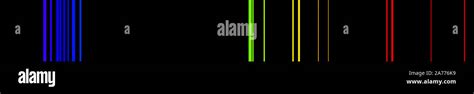
The Krypton emission spectrum has a range of applications, from laser technology to plasma diagnostics. The sharp spectral lines of Krypton make it an ideal candidate for spectroscopic analysis, where the emitted radiation can be used to determine the composition and properties of a sample. Additionally, the Krypton emission spectrum is used in lighting applications, such as in fluorescent lamps, where the emitted radiation is used to excite a phosphor coating, producing visible light.
Plasma Diagnostics
The Krypton emission spectrum can be used to diagnose the properties of a plasma, such as its temperature and density. By analyzing the spectral lines of Krypton, researchers can gain insights into the energy transitions that occur within the plasma, and hence determine its properties. This has applications in fields such as fusion research, where the properties of a plasma are critical to achieving controlled nuclear fusion.
The Krypton emission spectrum is also used in astronomical spectroscopy, where the emitted radiation can be used to determine the composition and properties of celestial objects, such as stars and galaxies. By analyzing the spectral lines of Krypton, astronomers can gain insights into the chemical composition and physical properties of these objects, and hence better understand the universe.
What is the significance of the Krypton emission spectrum in plasma diagnostics?
+The Krypton emission spectrum is significant in plasma diagnostics because it allows researchers to determine the properties of a plasma, such as its temperature and density. By analyzing the spectral lines of Krypton, researchers can gain insights into the energy transitions that occur within the plasma, and hence determine its properties.
How is the Krypton emission spectrum used in lighting applications?
+The Krypton emission spectrum is used in lighting applications, such as in fluorescent lamps, where the emitted radiation is used to excite a phosphor coating, producing visible light. The sharp spectral lines of Krypton make it an ideal candidate for spectroscopic analysis, and its emitted radiation can be used to produce a wide range of colors.
In conclusion, the Krypton emission spectrum is a complex and fascinating phenomenon that has a range of applications, from laser technology to plasma diagnostics. The sharp spectral lines of Krypton make it an ideal candidate for spectroscopic analysis, and its emitted radiation can be used to determine the composition and properties of a sample. By understanding the Krypton emission spectrum, researchers can gain insights into the energy transitions that occur within the atom, and hence develop new technologies and applications that exploit this phenomenon.